Novel control of Dengue fever
The spread of Dengue fever in northern Australia may be controlled by a bacterium that infects mosquitoes that harbor the virus, Australian and U.S. researchers report Aug. 25 in two papers published in the journalNature.
结果20多年前的工作by population biologist Michael Turelli, professor of evolution and ecology at UC Davis, and Ary Hoffmann, now at the University of Melbourne, Australia, who are among the coauthors of one of the newNaturepapers.
Turelli and Nick Barton of the Institute of Science and Technology, Austria, also describe the mathematical basis of thedengueelimination project in a paper to be published in the journalAmerican Naturalistin September.
Dengue fever is caused by fourvirus strainsspread by the mosquitoAedes aegypti. The disease causeshigh feverand has been called "breakbone fever" because of the joint aches and muscle pains it causes. Dengue viruses can also cause a potentially fatal disease, dengue hemorrhagic fever, in people who have previously been infected with a different strain of the virus.
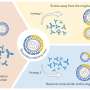
Dengue viruses are found throughout the tropics andsubtropicsand appear annually innorthern Australia. The researchers releasedmosquitoesinfected with the bacterial parasiteWolbachia, which suppresses the virus, and now report that theWolbachiaparasite spreads rapidly through the wild mosquito population.
"The results show we can completely transform local populations in a few months," Turelli said.
Wolbachiais transmitted byfemale mosquitoesto their offspring. A pair of infected mosquitoes produce slightly fewer eggs than an uninfected couple, but when an infected male mosquito mates with an uninfected female, she produces no eggs at all. That provides a big reproductive advantage to the spread ofWolbachia-infected mosquitoes, generation by generation.
"It's natural selection on steroids," Turelli said.
It turns out thatWolbachiaalso suppresses various other microbes living in the same mosquito – including the dengue virus. As these virus-resistant mosquitoes spread through the wild population, dengue transmission should dry up.
Turelli and Hoffmann first described what turned out to beWolbachiaspreading amongDrosophilaflies in California's Central Valley in 1991, and Barton developed much of the relevant mathematics in the late 1970s while trying to understand the genetics of grasshoppers in the French Alps. That basic research by Turelli, Hoffman and Barton provides the biological and mathematical basis for the dengue control strategy.
"At the time, none of us expected that this original research might contribute to human health. This is very exciting, once-in-a-lifetime opportunity," Turelli said. "We never thought this would turn into an eradication project."
The mathematics is complicated because whenWolbachiais rare, its spread through an insect population is disadvantaged because infected couples lay fewer eggs than uninfected. However, once the frequency of the infection crosses a certain threshold, there is a strong advantage to its spread.
Originally, Turelli and other researchers lead by Scott O'Neill at the University of Queensland, funded by the Bill & Melinda Gates Foundation, tried to useWolbachiato shorten the lifespan of Aedes so that the virus would not have the 12 days necessary to develop. However, that approach seems unlikely to work, based on the mathematics of the spread of that type ofWolbachia.
Instead, the team found thatWolbachiaitself suppresses certain viruses. The Gates Foundation is providing further funding to support release of infected mosquitoes in Australia, Vietnam and Thailand.