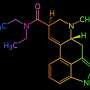
Tofacitinib ups rheumatoid arthritis treatment response
(HealthDay)—The addition of tofacitinib to rheumatoid arthritis (RA) treatment regimens improves patient response to non-biologic disease-modifying antirheumatic drugs (DMARDs), according to a study published in the Aug. 20 issue of theAnnals of Internal Medicine.
Joel Kremer, M.D., from Albany Medical College in New York, and colleagues conducted a one-year trial of tofacitinib in 792 patients with active RA despite non-biologic DMARD therapy seen at 114 centers in 19 countries. Patients were randomly assigned in a 4:4:1:1 ratio to oral tofacitinib (5 or 10 mg twice daily) or placebo advanced to tofacitinib (5 or 10 mg twice daily). Improvement was determined using American College of Rheumatology (ACR20) criteria; Disease Activity Score for 28-joint counts based on theerythrocyte sedimentation rate(DAS28-4 [ESR]) of less than 2.6; DAS28-4(ESR)-defined remission; and change in Health Assessment Questionnaire Disability Index (HAQ-DI) score.
The researchers found that, for the 5-mg and 10-mg tofacitinib groups, the mean treatment differences by ACR20 criteria compared with the combined placebo groups were 21.2 and 25.8 percent, respectively. The tofacitinib groups also had superior HAQ-DI scores (month three) and DAS28-4(ESR) less than 2.6 response rates (month six) compared to placebo. For patients receiving 5-mg tofacitinib, 10-mg tofacitinib, and placebo, the incidence rates ofserious adverse eventswere 6.9, 7.3, and 10.9 events per 100 patient-years of exposure, respectively. Additionally, in the tofacitinib groups, neutrophil counts decreased, hemoglobin and low- and high-density lipoprotein cholesterol levels increased, and serum creatinine levels had small increases.
"Tofacitinib improved disease control in patients with active RA despite treatment with non-biologic DMARDs, primarily methotrexate," the authors write.
This study was funded by Pfizer, the manufacturer of tofacitinib.
Copyright © 2013HealthDay. All rights reserved.