World's smallest, leadless heart pacemaker implanted at Ohio State
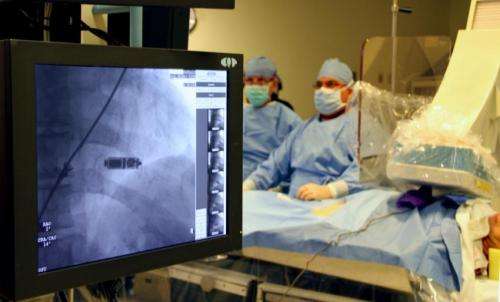
It's about the size of a large vitamin pill and, for the first time in Ohio, the smallest heart pacemaker available is being tested at The Ohio State University Wexner Medical Center.
医生在俄亥俄州立大学的理查德·m·罗斯的心Hospital recently implanted the tiny device in a Columbus woman as part of a global clinical trial to test its safety and effectiveness. Unlike conventional pacemakers, which require a chest incision and electrical leads that run through a vein to the heart, this device is wireless and is threaded through a catheter, then attached directly to the heart muscle.
"With this investigational device, the battery, the pacing electrodes, everything is in a little piece of metal sitting inside the heart. We believe that will eliminate a lot of risk for infection and complications," said Dr. John Hummel, a cardiologist and principal investigator of the trial at Ohio State.
If this transcatheter, leadless pacemaker technology works the way doctors hope, they say it could not only benefit patients, but the minimally invasive approach would be more efficient.
"I think this could be a significant development in pacing procedures. This could cut our procedure time by more than half," said Dr. Ralph Augostini, a cardiologist at Ohio State.
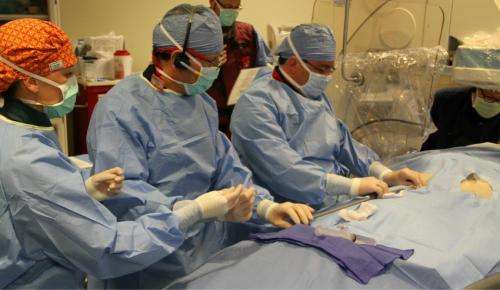
For now, the tiny pacemaker is being tested in people with bradycardia who need single chamber ventricular pacing. Bradycardia is a slow,irregular heart rhythmwhich prevents the heart from pumping enough blood into the body. This can cause fatigue, dizziness, shortness of breath and fainting.
A former librarian, 77-year-old Mary Lou Trejo of Columbus, had been suffering from atrial fibrillation for years. Herhearthad slowed, despite medication and other treatments to restore rhythm, so she was eager to be among the first in the United States to participate in this clinical trial.
"The newpacemakersounded so simple, and I have always thought research is important, so I thought this is a way I could contribute," Trejo said.
The trial will enroll 780 patients in 50 centers worldwide. Investigators are expected to report initial results later this year, once the first 60 patients have been followed for three months.