Stage IIIA non-small cell lung cancer survival rates improved when care includes four specific quality measures
Current guidelines from the National Comprehensive Cancer Network (NCCN) and American College of Chest Physicians (ACCP) recommend that operable patients with clinical Stage IIIA non-small cell lung cancer (NSCLC) should receive induction chemotherapy (with or without concurrent radiotherapy) followed by resection if there is no apparent progression of disease. While four quality measures have been identified as associated with improved overall survival, until now it has been unclear to what extent patients are actually receiving each of these measures as part of their care. A presentation at the 96th AATS Annual Meeting clearly demonstrates that survival rates increase as more quality measures are incorporated into patient care - but only 13% of eligible patients actually received all four measures.
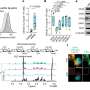
Analysis of data from the National Cancer Data Base (NCDB) demonstrates that thesurvival ratesof patients with Stage IIIA NSCLC who underwent surgery increased more than three-fold for those who received fourquality measuresas part of their care. The study, presented by Pamela Samson MD, MPHS, of Washington University in St. Louis at the 96th AATS Annual Meeting, also shows a wide variability in compliance with quality measures, with only 12.8% of almost 8,000 eligible patients having received all four interventions. The study highlights the importance of implementing these recommended steps into actual practice.
"Compliance with national recommendations regarding induction therapy and approach to surgical resection are crucial to optimizing long-term survival outcomes in clinical Stage IIIA NSCLC," explained lead author Dr. Samson.
研究人员分析了NCDB数据识别clinical Stage IIIA NSCLC patients who underwent surgical resection from 2006-2011. This database is a joint collaboration between the American College of Surgeons and the American Cancer Society and contains patient, tumor, and treatment data for approximately 70% of cancer patients receiving care at Commission on Cancer accredited centers. The four quality measures investigated were neoadjuvant multiagent chemotherapy, lobar (or greater) resection, sampling of at least 10 lymph nodes, and R0 resection, which means that the tumor has been removed to the extent that the margins are free of cancerous cells.
Data clearly indicated the benefit of including more quality measures into patient care. Overall median survival, which began at 12.7 months for patients who did not receive any of the measures, increased to 25.0 months for those receiving one measure, 31.4 months for those receiving two measures, 36.6 months for those receiving three measures and 43.5 months for receiving all four measures.
Between 2006 and 2010, 19% (10,304) of 54,069 patients with clinical Stage IIIA NSCLC underwent surgical resection. Among these patients, the most frequently missed measure was receipt of neoadjuvant multiagent chemotherapy (only approximately 30%), followed by sampling of at least 10 lymph nodes taken for only 40% of patients. Eighty-four percent received a lobectomy or greater resection and 87% obtained negative surgical margins. Despite the proven benefits of receiving all four quality measures as part of patient care, the investigators found that only 12.8% of individuals with clinical Stage IIIA NSCLC received all four interventions.
When the investigators looked at what variables were associated with receiving the most complete care, they found private insurance or Medicare status, higher education, and receiving care at an academic cancer or high-volume surgical center were associated with increased likelihood of receiving all four quality measures. Those who received fewer interventions tended to be older, non-Caucasian, and with multiple comorbidities.
"This analysis demonstrated that the majority of clinical Stage IIIA NSCLC patients are not receiving multiagent induction chemotherapy prior to resection. This is surprising, given the fact that the majority of patients in this study (80%) presented with clinical N2 disease," commented Dr. Samson. Noting that median overall survival was greater for patients who received multiagentinduction chemotherapycompared to those who did not, Dr. Samson emphasized that "this underscores the need for evaluation of the clinical Stage IIIA NSCLC patient by a multi-disciplinary group."
The authors noted that "while this study demonstrated that achieving these selected quality measures, both individually and collectively, was associated with improved overall survival, it also revealed that patient, institutional, and tumor factors independently influence whetherpatientsreceive these key quality measures." They hope that the findings from such national reviews of quality measure compliance will encourage individual institutions to evaluate their practice patterns as there may be variation both within and among cancer centers.
More information:“手术质量度量阶段iii a非小Cell Lung Cancer Are Associated with Improved Survival," by Pamela Samson, MD, MPHS, Traves D. Crabtree, MD, Clifford G. Robinson, MD, Daniel Morgensztern, MD, Stephen R. Broderick, MD, Daniel Kreisel, MD, PhD, A. Sasha Krupnick, MD, G. Alexander Patterson, MD, Bryan F. Meyers, MD, MPH, Varun Puri, MD, MSCI. Presentation at the 96th AATS Annual Meeting, May 14-18, 2016, Baltimore, MD, during the Plenary Scientific Session on May 16,aats.org/annualmeeting