Retaining one normal BRCA gene in breast, ovarian cancers influences patient survival
Determining which cancer patients are likely to be resistant to initial treatment is a major research effort of oncologists and laboratory scientists. Now, ascertaining who might fall into that category may become a little easier for physicians taking care of people withBRCA1/2mutations. Researchers in the Perelman School of Medicine at the University of Pennsylvania found a relationship between the genetics of tumors with germlineBRCA1/2mutations and whether the tumor retains the normal copy of theBRCA1/2基因,主要阻力共同的风险chemotherapy that works by destroying cancer cells' DNA. The team published their study this week inNature Communications.
Researchers estimate that 5 percent of breast cancers and 20 percent of ovarian cancers are attributable to germline mutations inBRCA1andBRCA2, the focus of the current study. Overall, 252,710 people will be diagnosed with breastcancerthis year and 40,610 will die of the disease, according to the National Cancer Institute. For ovarian cancer, NCI projects 22,440 new cases and 14,080 deaths.
There are many reasonspatientsmay be resistant to treatment—the immune system, the complex landscape of a tumor, or a patient's own genes can all play a role. Without explicitly looking for it, the Penn team found another mechanism of resistance to a standard treatment for patients with BRCA-associated cancers. "Our primary question was not aimed at evaluating resistance to therapy, but we did end up there," said senior author Katherine Nathanson, MD, deputy director of the Abramson Cancer Center, and director of Genetics at the Basser Center for BRCA.
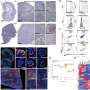
Her group evaluated the genetic profiles of 160 breast andovarian cancers与germline mutationsinBRCA1and BRCA2, in the largest study of these tumors to date. They were interested in determining what types of secondary, additional changes occur in primaryBRCA1/2germline mutation-associated cancers that might act in concert with mutantBRCA1andBRCA2to drive the cancers.
The team evaluated how frequently the non-mutated version of the gene lost its function in concert with the originalBRCA1/2germline mutation-associated cancers. In oncology terms, this double-hit status is called "loss of heterozygosity," or LOH, to signify that both versions (one inherited from mother, one from father) of the normal BRCA gene have been hobbled.
Historically, it had been thought that all tumors associated with germlineBRCA1/2mutations lose the second version of the gene, or LOH. The investigators were surprised to find that was not the case in a surprisingly large percentage of patients. In addition, they found that other genetic and clinical features of patients whose tumors did not undergo LOH (LOH-negative) were significantly different from those that did undergo LOH (LOH-positive).
Notably, they evaluated the overall survival of patients with ovarian tumors with and without loss of heterozygosity. LOH-negative status was associated with worse overall survival in ovarian cancer patients treated with platinum chemotherapy, with a median of 39 months, compared to 71 months in the LOH-positive group who received the same treatment.
The researchers believe the patients with LOH-negative tumors (those with one working copy ofBRCA1orBRCA2and the other copy carrying the germline mutation) had tumor cells that could still repair the chemotherapy-induced DNA damage in order to survive. In contrast, the investigators surmise that the LOH-positive group (with both gene copies disabled) responded better to the same therapy because theirtumorcells died more readily.
"Identifying the LOH status ofBRCA1/2carriers may be useful to predict who might be at risk for primary resistance to DNA-damaging agents such as platinum, which has important implications for treatment of patients with these mutations," said the study's first author Kara N. Maxwell, MD, PhD, an instructor of Hematology/Oncology. "We only need to determine the LOH at a specific gene's location, which is more cost effective than sequencing a patient's whole genome, for example, and compatible with standard testing."
通过观察一个人的个体遗传学和type of cancer, the Penn team hopes to be able to better tailor care soon after an initial diagnosis to improve survival. Nathanson surmises that knowing a person's LOH status could guide treatment decisions. She suggests that certain drugs already in today's cancer treatment arsenal will likely work for patients who are at risk for resistance due to their LOH genetics; however, it's a matter of choosing the right one.