Bacteria in the gut modulates response to immunotherapy in melanoma
Bacteria that live in the human digestive tract can influence how cancer responds to immunotherapy, opening a new avenue for research to improve treatment, a team led by researchers at The University of Texas MD Anderson Cancer Center reports in the journalScience
Patients with metastatic melanoma treated with anti-PD1 checkpoint blockade have their disease controlled longer if they have a more diverse population of bacteria in the gut or an abundance of certain types of bacteria, according to the team's analysis offecal samplesto assess patients' gut microbiomes.
“你可以改变你的微生物,这真的不是that difficult, so we think these findings open up huge new opportunities," said study leader Jennifer Wargo, M.D., associate professor of Surgical Oncology and Genomic Medicine. "Our studies in patients and subsequent mouse research really drive home that our gut microbiomes modulate both systemic and anti-tumor immunity."
Wargo and colleagues are working with the Parker Institute for Cancer Immunotherapy to develop a clinical trial that combines checkpoint blockade with microbiome modulation.
Research has shown that a person's microbiome is a modifiable risk factor that can be targeted by diet, exercise, antibiotic or probiotic use or transplantation of fecal material, said lead co-first author Vancheswaran Gopalakrishnan, Ph.D.
Immune checkpoint blockade drugs that free the body's own immune system to attack cancercellshelp around 25 percent of metastatic melanoma patients, and those responses are not always durable. Research focuses on extending the impact of these drugs.
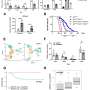
To assess the impact of the microbiome, Wargo and colleagues analyzed buccal swabs - tissue samples from inside the cheek—and fecal samples of patients treated with anti-PD1 therapy that blocks the PD1 protein on T cells, which acts as a brake on the immune system. They conducted 16S rRNA and whole genome sequencing to determine diversity, composition and functional potential of the buccal and fecal microbiomes.
而团队没有发现实质性的差异response or progression based on buccal samples, analysis of fecal samples of 30 patients who responded to treatment and 13 who did not told a different story.
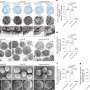
- Patients with higher diversity of bacteria in their digestive tract had longer median progression-free survival (PFS), defined at the time point where half of studied patients have their disease progress. While the patient group with high diversity had not reached median PFS (more than half had not progressed), those with intermediate and low diversity had median PFS of 232 and 188 days respectively.
- Notable compositional differences existed in the gut microbiome of patients who responded versus those who did not, with the Ruminococcaceae family enriched in responders and the Bacteroidales order enriched in non-responders. Patients who had a high abundance of the genus Faecalibacterium (of the Ruminococcaceae family and Clostridiales order) in their gut had significantly prolonged PFS (median not reached), compared to patients who had a low abundance (median PFS of 242 days)
- Abundance of Bacteroidales was associated with more rapid disease progression, with high abundance within the gut microbiome associated with significantly reduced PFS (median 188 days), compared to low abundance (median PFS of 393 days).
Additional analysis showed that responding patients with high levels of the beneficial Clostridiales/Ruminococcaceae had greater T cell penetration into tumors and higher levels of circulating T cells that kill abnormal cells. Those with abundant Bacteriodales had higher levels of circulating regulatory T cells, myeloid derived suppressor cells and a blunted cytokine response, resulting in dampening of anti-tumor immunity.
A favorable microbiome also was associated with increased antigen processing and presentation by the immune system at the tumor site.
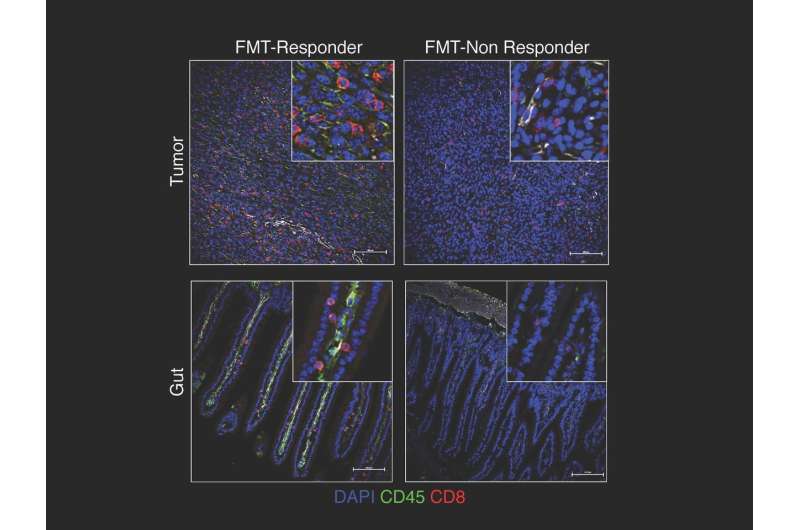
To investigate causal mechanisms, the team transplanted fecal microbiomes from responding patients and non-responding patients via fecal microbiome transplant (FMT) into germ-free mice. Those receiving transplants from respondingpatientshad significantly reduced tumor growth as well as higher densities of beneficial T cells and lower levels ofimmune suppressive cells. They also had better outcomes when treated with immune checkpoint blockade.
Wargo and colleagues note that there is still much to learn about the relationship between themicrobiomeand cancer treatment, so they urge people not to attempt self-medication with probiotics or other methods.
More information:V. Gopalakrishnan el al., "Gut microbiome impacts response to anti-PD-1 immunotherapy in melanoma patients,"Science(2017).science.sciencemag.org/lookup/ … 1126/science.aan4236