The first effective therapy against glioblastoma achieved by attacking telomeres
The Telomere and Telomerase Group at the Spanish National Cancer Research Centre (CNIO) has shown that it is possible to block the growth of human and murine glioblastoma in mouse models by blocking the TRF1 protein, an essential component of the telomere-protective complex known as shelterin. The study, published inCancer Cell, describes a new and promising way to combat this type of brain tumour, considered one of the most lethal and difficult to treat, by attacking its ability to regenerate and divide immortally.
The average life expectancy of patients withglioblastomais about 14 months. This common braintumourcan evade and overcome the limited therapeutic options that exist today to treat it. It is particularly known for its ability to regenerate, as the tumour contains a subset of cells with characteristics that are similar to stem cells, called glioblastoma stem cells (GSCs). One of these cells is capable of reproducing the entire tumour.
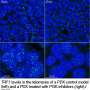
These GSCs cells are the cornerstone of glioblastoma and one of its identifying features. One of their characteristics is that they have very high levels of the telomeric protein TRF1, which in addition to being essential for protecting telomeres, is required to maintain the capacity of these cells to regenerate the tumour.
"We know that TRF1 is largely expressed in stem cells, so we thought it would be interesting to see what would happen in tumours that had a strong tumour stem cell nature if we blocked TRF1," explains Maria A. Blasco, head of the Telomeres and Telomerase Group and senior author of the paper. Glioblastoma is clearly a type of tumour that could benefit from blocking TRF1 owing to the ability of its glioma stem cells to regenerate the tumours after current treatments.
"The first thing we saw was that TRF1 is highly overexpressed in both mouse and human glioblastomas, which indicated that by blocking it we could perhaps impair its growth," says Leire Bejarano, a member of Blasco's group and first author of the paper.
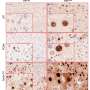
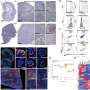
Consequently, Blasco, Bejarano and colleagues started working with mouse models. They removed TRF1 during the initiation of the tumour, as well as blocked it once the glioblastomas had already formed. "Both strategies led to a significant increase in the survival rate of the mice with glioblastomas," said Bejarano. In the first case, the increase in survival was of 80 percent and in the case of existing tumours, the increase in survival was 30 percent.
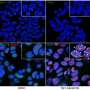
By studying the mechanisms by which TRF1 inhibition limitedtumour growth, they found that inhibiting TRF1 caused a reduction in the proliferation and in the stem properties of glioma stem cells. This was triggered by an increase in DNA damage at the telomeres, which resulted from the destruction of glioblastoma telomeres. In the end, they prevented the tumour cells from continuing to multiply.
After the success in the mouse models, they began to work with human tumourcells. This required grafting glioblastomastem cellsderived from human patients into mice and treating them with a series of compounds developed at CNIO that inhibit TRF1 and which mechanism of action has been described recently by the same CNIO group. When compared with the animals treated with TRF1 inhibitors with those treated with a placebo, those treated with the TRF1 inhibitor displayed a reduction in the growth and size of the tumours, accompanied by an 80 percent decrease in tumour TRF1 levels and an increase in the survival rate.
除了observ anti-tumoural属性ed, blocking TRF1 appears to be safe because did not affect the olfactory and neuromuscular functions, nor the memory, of the mice. This strengthens the idea that we now have a new therapeutic window to inhibit TRF1 in this type ofbrain tumour.
"It has a major therapeutic effect on glioblastoma," says Blasco.
"We see that inhibiting TRF1 is an effective strategy for treating glioblastoma both by itself and in combination with current radiation and temozolomide therapies," explained the authors in their report.
The next step, already underway, is to verify the effectiveness of the TRF1 inhibitors developed at CNIO in combination with other drugs that are already being used in the clinic.
更多的信息:Inhibition of the TRF1 telomere protein impairs tumor initiation and progression in glioblastoma multiforme mouse models and in patient-derived xenografts. Leire Bejarano, Alberto J. Schuhmacher, Marinela Méndez, Diego Megías, Carmen Blanco-Aparicio, Sonia Martínez, Joaquín Pastor, Massimo Squatrito and Maria A. Blasco,Cancer Cell2017,DOI: 10.1016/j.ccell.2017.10.006