Expansion stress enhances growth and migration of breast cancer cells
Expansion stress can have an alarming impact on breast cancer cells by creating conditions that could lead to dangerous acceleration of the disease, an interdisciplinary team of University of Alabama at Birmingham researchers has found.
Asbreast tumorsgrow, biomechanical forces in thetumor microenvironment, or TME, cause elevated compression at the tumor interior, tension at the periphery and altered interstitial fluid flow—promoting aggressive growth, invasion and metastasis. Biomechanical forces also may modulate theimmune responsethrough cancer cell-immune cell crosstalk.
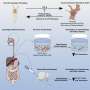
The UAB researchers—Joel Berry, Ph.D., associate professor in the Department of Biomedical Engineering; Jessy Deshane, Ph.D., associate professor in the UAB Department of Medicine's Division of Pulmonary, Allergy and Critical Care Medicine; Roy Koomullil, Ph.D., associate professor in the Department of Mechanical Engineering; and Selvarangan Ponnazhagan, Ph.D., professor in the Department of Pathology—created a novel, tissue-engineered, three-dimensional breast cancer mimetic system.
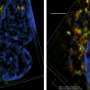
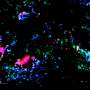
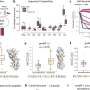
This system recapitulates the in vivo growth ofbreast cancer cellsin the presence of tumor-associated fibroblasts,endothelial cellsand immune cells, within a physiologically relevant extracellular matrix. The researchers found that biomechanical forces significantly altered the proteome of breast cancer cells and enhanced exosome production. Tumor cell-secreted exosomes, one of the intercellular mediators of signaling in the TME, are now recognized as key regulators of tumor progression.
In the study, the exosomes directly promoted aggressive tumor cell growth, induced immune suppression and altered immune cell polarization in the TME. Furthermore, the researchers recently engineered an oscillatory compression device for real-time application of biomechanical force on orthotopic mammary tumors in vivo, which allowed them to observe exosome-mediated immunosuppression and aggressive tumor growth in mice.
Preliminary analyses of exosome migration, immune cell uptake and polarization superimposed onto a novel computational algorithm indicated the significance of exosome concentration gradient and time in predicting the kinetics of protumorigenic events, linking biomechanical force, exosome release by tumor cells, exosome uptake and polarization ofimmune cellsin the TME.
The study, "Mechanical strain induces phenotypic changes in breast cancer cells and promotes immunosuppression in the tumor microenvironment," is published in the journalLaboratory Investigation.
Explore further