Cortex-wide variation of neuronal cellular energy levels depending on the sleep-wake states
It is assumed that the brain has homeostatic mechanisms to prevent the depletion of cellular energy, required for all cellular activities. For example, the blood flow increases, and oxygen and glucose are actively delivered in the brain region in which neural firing activity occurs. Besides, the cerebral blood flow and glucose uptake into the cells fluctuate accompanying the variations of cellular activities in the brain across the sleep-wake states of animals. Under these brain energy homeostatic mechanisms, it is assumed that the cellular energy status in the brain could be maintained constant in all physiological conditions including across the sleep-wake states of animals. However, this has not been experimentally proven.
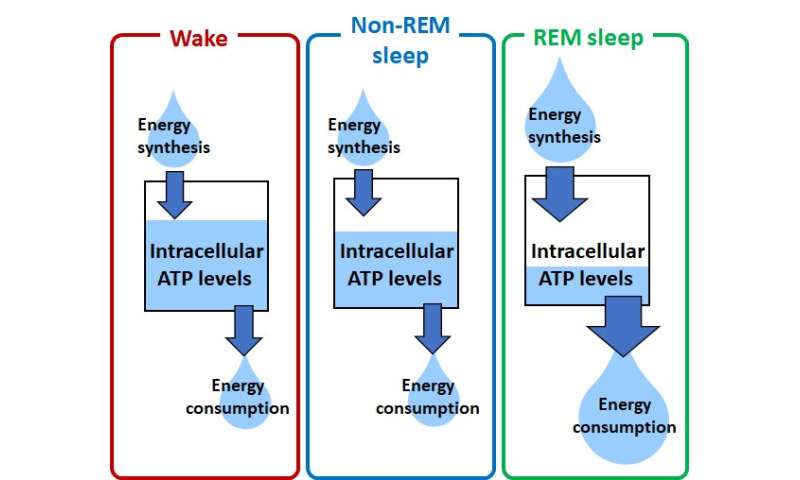
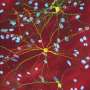
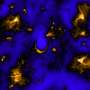
To investigate whether the cellular energy status in the brain of living animals is always constant or variated, the researchers measured the neuronal intracellular concentration of adenosine 5'-triphosphate (ATP), the major cellular energy metabolite, using a fluorescent sensor in the brain of living mice. Using anoptical fiberand wide-field microscopy, they showed a cortex-wide variation of cytosolic ATP levels in thecortical neuronsdepending on the sleep-wake states of animals: The ATP levels were high during the waking state, decreased during non-REM sleep, and profoundly decreased during REM sleep. On the other hand,cerebral blood flow, as a metabolic parameter forenergy supply, slightly increased during non-REM sleep and greatly increased during REM sleep, compared with the waking state. The reduction in neuronal ATP levels was also observed under general anesthesia in mice and response to local brain electrical stimulation for neuronal activation, whereas the hemodynamics was simultaneously enhanced.
Since the neuronal ATP levels increase throughout the cortex in the waking state, which is when the cellular energy demand increases, brain mechanisms for energy modulation could increase the neuronal ATP levels in a cortex-wide manner in response to the sleep-to-wake transition of animals. Meanwhile, the great reduction of neuronal ATP levels during REM sleep despite a simultaneous increase of cerebral hemodynamics for energy supply suggests negative energy balance in neurons, which could be due to REM sleep-specific promotion of energy-consuming activities such as heat production. The significant reduction of ATP levels in the cortical neurons during REM sleep is expected to use as a novel biomarker of REM sleep. Eventually, cerebral energy metabolism may not always meet neuronal energy demands, consequently resulting in physiological fluctuations of intracellular ATP levels in neurons.