Response to COVID-19 vaccines varies widely in blood cancer patients
Patients with a type of blood cancer called multiple myeloma had a widely variable response to COVID-19 vaccines—in some cases, no detectable response—pointing to the need for antibody testing and precautions for these patients after vaccination, according to a study that will be published inCancer Cellthis week.
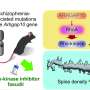
Mount Sinai researchers found that multiple myelomapatientsmount variable and sometimes suboptimal responses after receiving the Pfizer-BioNTech or Moderna COVID-19 vaccines. Almost 16 percent of these patients developed no detectibleantibodiesafter bothvaccine doses. These findings may be relevant to othercancer patientsundergoing treatment and to immunocompromised patients.
"This study underscores the need for routine blood tests on multiple myeloma patients after vaccination to understand their risk and potential need to continue wearing masks and socially distance until the pandemic wanes," said co-lead author Samir Parekh, MD, Director of Translational Research in Multiple Myeloma at The Tisch Cancer Institute at Mount Sinai and Professor of Medicine (Hematology and Medical Oncology), and Oncological Sciences, at the Icahn School of Medicine at Mount Sinai. "This also calls forclinical trialsto study the use of prophylactic therapies, likemonoclonal antibodies, to mitigate COVID-19 risk or to use of different vaccines or booster vaccinations in these patients."
COVID-19 preventi疫苗是非常有效的ng severe infections or death, but patients with multiple myeloma are immunocompromised and often on immunosuppressive therapy, and preliminary reports showed that the vaccines evoked a lower and delayed response in them compared to healthy people. This study was the first to analyze a large group of patients with multiple myeloma after completing both doses of vaccination and to compare their antibody response to a control group of healthy people.
"As we continue to reopen the country, it is important for people with immune system disorders, including multiple myeloma, to work with their doctors and to understand their response to their COVID-19 vaccines due to the varied antibody responses to the vaccines we see in this study," said co-lead author Ania Wajnberg, MD, Director of Clinical Antibody Testing at The Mount Sinai Hospital.
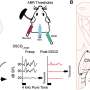
重复测量抗体从病人的面前first vaccine dose until 60 days after the second vaccination showed delayed and suboptimal responses, particularly in patients with multiple myeloma who had not contracted COVID-19 before their vaccinations. Patients on active cancer treatment had significantly lower antibody levels after two vaccine doses than multiple myeloma patients who were not on treatment at the time of vaccination.
Researchers analyzed the antibody levels of 320 multiple myeloma patients, 260 of whom received two doses of COVID-19 vaccinations, and found that 15.8 percent had undetectable antibodies. The multiple myeloma patients who had had COVID-19 before vaccination showed immune responses that were 10 times higher than those who had not.
Pointing to the importance of the study, 10 multiplemyelomapatients who received at least one dose of COVID-19vaccinedeveloped COVID-19 during the study period. Four needed to be hospitalized with severe symptoms and one of them died.