Potential safety concern for Guillain-Barré syndrome identified for Johnson & Johnson vaccine
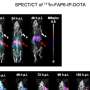
(HealthDay)—Receipt of the Ad26.COV2.S (Janssen/Johnson & Johnson) COVID-19 vaccine is associated with a potential small, but statistically significant, safety concern for Guillain-Barré syndrome (GBS), according to a study published online Oct. 7 in theJournal of the American Medical Association.
艾米丽珍吸引,医学博士,公共卫生学硕士,从美国食品nd Drug Administration in Silver Spring, Maryland, and colleagues examined reports of presumptive GBS received in the Vaccine Adverse Event Reporting System (VAERS) following Ad26.COV2.S vaccination.
The researchers identified 130 reports of presumptive GBS in the VAERS following Ad26.COV2.S vaccination as of July 24, 2021. The median time to onset of GBS was 13 days following vaccination; 105cases(81.4 percent) and 123 cases (95.3 percent) began within 21 and 42 days, respectively. Overall, 121 reports (93.1 percent) were serious, including one death. The estimated crude reporting rate was one case of GBS per 100,000 doses administered based on about 13,209,858vaccinedoses administered to adults in the United States. For the 42-day window, the overall estimated observed-to-expected rate ratio was 4.18. In the worst-case scenario analysis for adults aged 18 years and older, this corresponded to an estimated absolute rate increase of 6.36 per 100,000 person-years (about 8.36 cases per 100,000 person-years compared with a background rate of about two cases per 100,000 person-years).
"The absolute risk of GBS, both in the background population (≈2 per 100 000), and following Ad26.COV2.S vaccination (130 reports per ≈13 million vaccinations), is extremely small and far lower than the risk of COVID-19," the authors write.
更多的信息:Abstract/Full Text
Copyright © 2021HealthDay. All rights reserved.