This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Can controlling retinoic acid be a key to preventing infections in the gut?

A team of scientists from the Renaissance School of Medicine (RSOM) at Stony Brook University has identified a distinct role of retinoic acid, a metabolite of vitamin A, during the immune response of the gut. This finding, detailed in a paper published in theJournal of Experimental Medicine, and highlighted in a broader piece in the journal, could help lead to ways to control the retinoic acid response and therefore be used as a therapy or for vaccine development against infection or even to treat GI tumors.
Led by Brian Sheridan, Ph.D., Associate Professor in the Department of Microbiology and Immunology and Center for Infectious Diseases, the study involves basic research that centers on unraveling the factors that control the generation of cytotoxic memory CD8 T cells, which are an important arm of the body's anti-pathogen immune response as they kill pathogen-infected cells and produce anti-pathogencytokines. In fact, memory CD8 T cells provide long-lived and frontline protection at barrier tissues, highlighting their importance in vaccine design.
To date scientists have known that retinoic acid in the gut-draining lymph nodes promotes effector CD8 T cell migration to the intestines, enhancing the immune response. Additionally, vitamin A deficiency is associated with increased infections and poor vaccine efficiency.
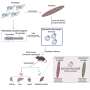
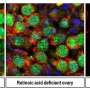
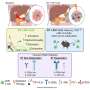
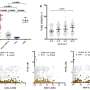
Sheridan and his co-authors, including Zhijuan Qiu, Ph.D., a post-doctoral fellow in the department, identified a new role for retinoic acid, which is a key part of the immune process in the gut. They demonstrated in the lab that T cell activation in gut-associated lymph nodes regulates memory CD8 T cell differentiation in the intestine. They also demonstrated in contrast that T cells activated at other sites were impaired in the ability to differentiate into memory CD8 T cells after entry into the intestine.
During this process, they demonstrated that activation within the gut-associated lymph nodes, but not in other sites, promotes intestinal memory CD8 T cell development and that retinoic acid signals provided during this window of T cell activation in the lymph nodes enhances intestinal memory CD8 T cell development to a wider degree.
“我们的研究强调了基本T的新角色cell activation on the generation of the intestinal memory CD8 T cells that appears distinct from other barrier sites like the lungs and skin," summarizes Sheridan. "Remarkably, we can alter intestinal T cell development by promoting or limiting retinoic acid signals during T cell activation, independent of the role of retinoic acid on T cell migration."
Because the research team was able to replicate this limiting or promoting of retinoic acid signals in the gut, they believe that manipulatingretinoic acidsignals during T cell activation may provide a strategy for clinicians to promote or limit intestinal CD8 T cells to improve vaccine outcomes or limit immunopathology.
More information:Zhijuan Qiu et al, Retinoic acid signaling during priming licenses intestinal CD103+ CD8 TRM cell differentiation,Journal of Experimental Medicine(2023).DOI: 10.1084/jem.20210923
Maximilian Heeg et al, License to kill: Retinoic acid programs T cells for tissue residency,Journal of Experimental Medicine(2023). DOI: 10.1084/jem.20230161 ,doi.org/10.1084/jem.20230161