This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
How do we know if our brain is capable of repairing itself?
Is our brain able to regenerate? And can we harness this regenerative potential during aging or in neurodegenerative conditions? These questions sparked intense controversy within the field of neuroscience for many years. A new study from the Netherlands Institute for Neuroscience shows why there are conflicting results and proposes a roadmap on how to solve these issues.
The notion of exploiting the regenerative potential of thehuman brainin aging orneurological diseasesrepresents a particularly attractive alternative to conventional strategies for enhancing or restoringbrain function, especially given the current lack of effective therapeutic strategies in neurodegenerative disorders like Alzheimer's disease.
The question of whether the humanbraindoes possess the ability to regenerate or not has been at the center of a fierce scientific debate for many years and recent studies yielded conflicting results. A new study from Giorgia Tosoni and Dilara Ayyildiz, under the supervision of Evgenia Salta in the laboratory of Neurogenesis and Neurodegeneration, critically discusses and re-analyzes previously published datasets. How is it possible that we haven't yet found a clear answer to this mystery?
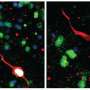
Previous studies in which dividingcellswere labeled in postmortem human brain, showed that new cells can indeed arise throughout adulthood in the hippocampus of our brain, a structure that plays an important role in learning and memory, and is also severely affected in Alzheimer's disease. However, other studies contradict these results and cannot detect the generation of new brain cells in this area.
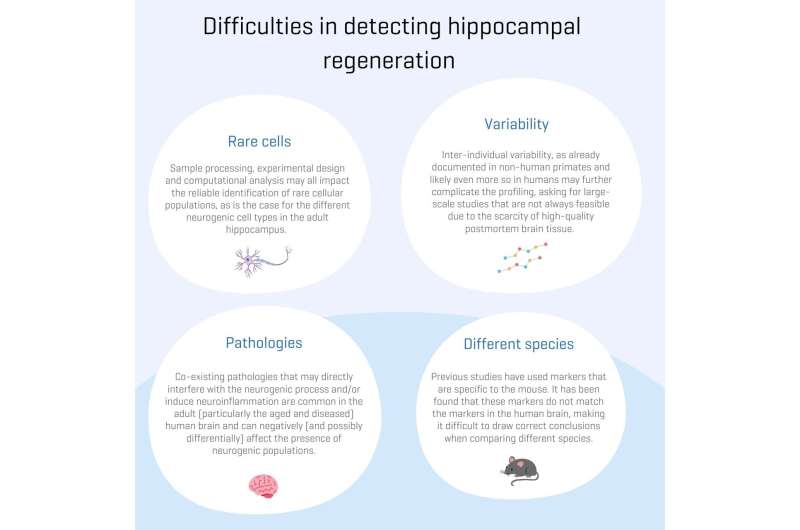
Both conceptual and methodological confounders have likely contributed to these seemingly opposing observations. Hence, elucidating the extent of regeneration in the human brain remains a challenge.
New state-of-the-art technologies
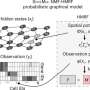
Recent advances in single-cell transcriptomics technologies have provided valuable insights into the different cell types found inhuman brainsfrom deceased donors with different brain diseases. To date, single-cell transcriptomic technologies have been used to characterize rare cell populations in the human brain. In addition to identifying specific cell types, single-nucleus RNA sequencing can also explore specific gene expression profiles to unravel full the complexity of the cells in the hippocampus.
The advent of single-cell transcriptomics technologies was initially viewed as a panacea to resolving the controversy in the field. However, recent single-cell RNA sequencing studies in human hippocampus yielded conflicting results.
Two studies indeed identifiedneural stem cells, while a third study failed to detect any neurogenic populations. Are these novel approaches—once again—failing to finally settle the controversy regarding the existence of hippocampal regeneration in humans? Will we eventually be able to overcome the conceptual and technical challenges and reconcile these -seemingly- opposing views and findings?
Technical issues
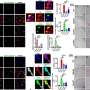
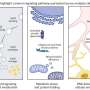
In this study, the researchers critically discussed and re-analyzed previously published single-cell transcriptomics datasets. They caution that the design, analysis and interpretation of these studies in the adult human hippocampus can be confounded by specific issues, which ask for conceptual, methodological and computational adjustments. By re-analyzing previously published datasets, a series of specific challenges were probed that require particular attention and would greatly profit from an open discussion in the field.
Giorgia Tosoni says, "We analyzed previously published single-cell transcriptomic studies and performed ameta-analysisto assess whether adult neurogenic populations can reliably be identified across different species, especially when comparing mice and humans. The neurogenic process in adult mice is very well characterized and the profiles of the different cellular populations involved are known."
"These are actually the same molecular and cellular signatures that have been widely used in the field to also identify neurogenic cells in the human brain. However, due to several evolutionary adaptations, we would expect the neurogenesis between mice and humans to be different. We checked the markers for every neurogenic cell type and looked at the amount of marker overlap between the two species."
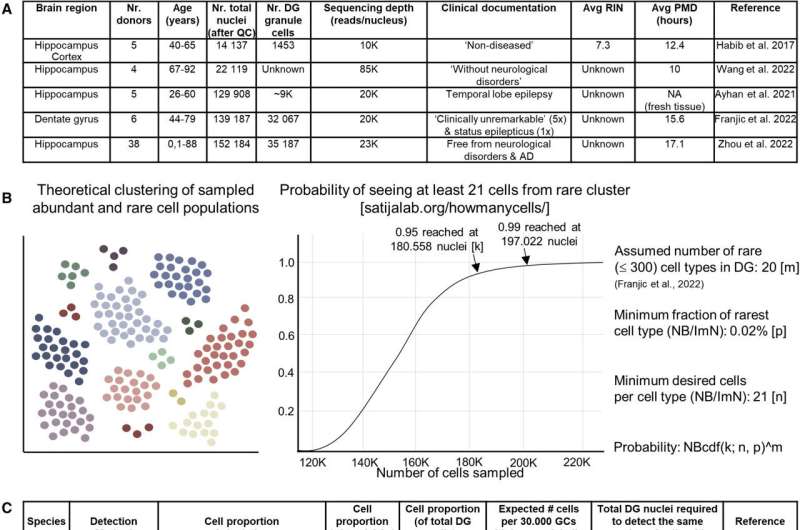
"We found very little, if no, overlap between the two, which suggests that the mouse-inferred markers we have been long using may not be suitable for the human brain. We also discovered that such studies require enough statistical power: if regeneration of neuronal cells does happen in the adult human brain, we expect it to be quite rare."
"Therefore, enough cells would need to be sequenced in order to identify those scarce, presumably neurogenic populations. Other parameters are also important, for example the quality of the samples. The interval between the death of the donor and the downstream processing is critical, since the quality of the tissue and of the resulting data drops over time."
Reproducibility is key
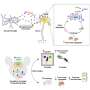
Dilara Ayyildiz说:“这些新技术,when appropriately applied, offer a unique opportunity to map hippocampal regeneration in the human brain and explore which cell types and states may be possibly most amenable to therapeutic interventions in aging, neurodegenerative and neuropsychiatric diseases. However, reproducibility and consistency are key. While doing the analysis we realized that some seemingly small, but otherwise very critical details and parameters in the experimental and computational pipeline, can have a big impact on the results, and hence affect the interpretation of the data."
"Accurate reporting is essential for making these single-cell transcriptomics experiments and their analysis reproducible. Once we re-analyzed these previous studies applying common computational pipelines and criteria, we realized that the apparent controversy in the field may in reality be misleading: with our work we propose that there may actually be more that we agree on than previously believed."
这项研究发表在《华尔街日报》Neuron.
More information:Giorgia Tosoni et al, Mapping human adult hippocampal neurogenesis with single-cell transcriptomics: Reconciling controversy or fueling the debate?,Neuron(2023).DOI: 10.1016/j.neuron.2023.03.010