This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Study: Conventional radiotherapy should remain standard of care for localized vertebral metastases of the spine
结果NRG肿瘤学RTOG 0631临床trial comparing stereotactic vs. conventional radiotherapy for localized vertebral metastases of the spine did not meet its primary endpoint. Data from the study suggests that radiosurgery was not considered superior in terms of pain responses at 3 months following treatment, and even displayed worse pain response, than the conventional external beam radiotherapy (cEBRT). These results were recently published in theJAMA Oncology.
cEBRT is currently the standard of care for treating spinal metastases because of its efficacy, butpain relieffor patients who received this treatment was approximately 50-60% and only lasted for a median duration of four months. Due to the advancements in Stereotactic radiosurgery (SRS) techniques, NRG-RTOG 0631 sought to determine whether this approach lessened pain or extended the time with pain relief for patients on the trial.
SRS has previously demonstrated safety and feasibility for treating patients with 1 to 3 sites of vertebral metastases in the Phase II portion of NRG-RTOG 0631. Phase III was designed to assess patient-reported pain relief at 3 months and treatment safety in the long-term follow-up of the trial.
Patients with 1 to 3 vertebral metastases enrolled onto NRG-RTOG 0631 were randomly assigned to receive either SRS or cEBRT. Patients on the SRS arm received a single dose 16 or 18 Gy delivered to the involved vertebral level or levels only, not including any additional spine levels, and patients on the cEBRT arm were treated with 8 Gy delivered to the involved vertebra plus one additional vertebra above and below.
The primary endpoint was patient-reported pain response defined as greater than or equal to a 3-point improvement on the Numerical Rating Pain Scale (NRPS) without worsening pain at the secondary site or the use of pain medication at 3 months.
Secondary endpoints of this trial included long-term pain control, toxicity, quality of life, and long-term effects on vertebral bone and spinal cord.
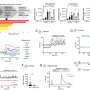
The NRG-RTOG 0631 baseline mean pain score +/- standard deviation at the index vertebra was 6.06±2.61 in the SRS arm and 5.88±2.41 in the cEBRT arm. Pain response at 3 months favored the cEBRT arm (41.3% for SRS vs. 60.5% for cEBRT; difference=-19 percentage points, 95% confidence interval [CI]: -32.9, -5.5, one-sided p=0.99, two-sided p=0.01).
Zubrod score was a significant factor influencingpainresponse. There were no differences in the proportion of acute or late adverse effects. Vertebral compression fractures at 24 months were19.5% with SRS and 21.6% with cEBRT (p=0.59). There were no spinal cord complications reported at 24 months.
"It is important to note that NRG-RTOG 0631 is the first phase II/III multicenter trial assessing the safety and efficacy of SRS for the treatment of vertebral metastases," stated Samuel Ryu, MD, of the Stony Brook University Medical Center and the lead author of the NRG-RTOG 0631 manuscript. "The data collected from this trial could also inform future trials, especially those assessing SRS for epidural tumor decompression,spinal cordcompression, and treating oligometastases where durable local tumor control improves survival."
More information:Samuel Ryu et al, Stereotactic Radiosurgery vs Conventional Radiotherapy for Localized Vertebral Metastases of the Spine,JAMA Oncology(2023).DOI: 10.1001/jamaoncol.2023.0356