This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Existing drugs prevent Alzheimer's disease-related cognitive impairment in mice, new research shows
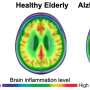
Changes in blood vessels in the brain linked to the build-up of a sticky protein known as amyloid beta are a hallmark of early-stage Alzheimer's disease. As amyloid accumulates on the walls of vessels, brain cells lose nutrients and oxygen, becoming inflamed and dysfunctional. Over time, this gives rise to cerebral amyloid angiopathy (CAA), a major cause of aging-related cognitive decline.
Reversing the effects of CAA and neuroinflammation could bring significant benefits for individuals at risk of Alzheimer's disease—and now, new research at the Lewis Katz School of Medicine at Temple University brings that hope within reach. In experiments carried out in mice, the Temple scientists show that drugs known as carbonic anhydrase inhibitors (CAIs), already FDA-approved for other conditions, such as glaucoma and high-altitude sickness, promote the clearance ofamyloid betafrom blood vessels andglial cells, which control brain inflammatory processes. In doing so, CAIs not only reduce inflammation and restore cell function but also prevent cognitive impairment.
The study took place in the lab of Silvia Fossati, Ph.D., Associate Professor of Neural Sciences and Cardiovascular Sciences at the Lewis Katz School of Medicine at Temple University, and is the first to test the FDA-approved CAIs acetazolamide and methazolamide in animals with cerebrovascular alterations mimicking those of CAA and Alzheimer's disease in humans. The results appeared online inAlzheimer's & Dementia: The Journal of the Alzheimer's Association.
"Cognitive impairment is closely associated with damage to blood vessels and inflammation in the brain, which are among the main causes, but also consequences, of amyloid accumulation," explained Dr. Fossati, who is also Associate Director of the Alzheimer's Center at Temple's Katz School of Medicine.
"We wanted to see if we could prevent cerebrovascular dysfunction, inflammation and improve cognitive function by therapeutically improving the health of vascular and glial cells, thereby facilitating the removal of amyloid from blood vessels and the brain tissue."
In previous work, Dr. Fossati and colleagues found that amyloid accumulation causes mitochondria—the energy-generating powerhouses of cells—to function abnormally and that this process is linked to activity of the enzyme carbonic anhydrase. "We also know from experiments in cells that CAIs suppress mitochondrial dysfunction and cell death induced by amyloid," Dr. Fossati said. "But whether these same effects occur in vivo has been unknown."
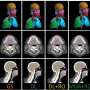
To test CAIs in vivo, Dr. Fossati and colleagues used a mouse model in which animals, as they age, exhibit increasing levels of human amyloid protein in the brain. As amyloid deposits accumulate in the brain vasculature, the animals begin to show signs of cerebrovascular dysfunction, similar to the way in which humans develop signs of CAA.
The animals were treated with either acetazolamide or methazolamide from about 8 months of age, when signs of amyloid pathology first emerge in these animals, until about 15 or 16 months, when advanced cognitive impairment is present.
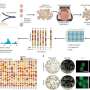
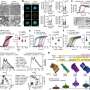
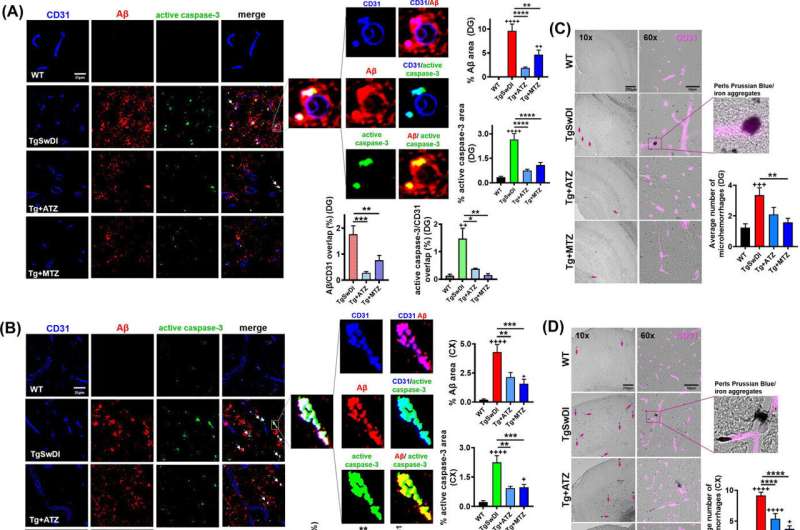
When the researchers examined brain tissue from CAI-treated mice, they found significant reductions in amyloid in the cerebral vasculature and in glial cells. They also found that glial cells andblood vesselswere overall healthier and had better amyloid-clearing capacity compared to untreated animals.
"Both acetazolamide and methazolamide were highly effective in reducing amyloid deposition and in improving cerebrovascular function," Dr. Fossati said. "Our behavioral studies showed that, as Alzheimer's pathology decreased, CAI-treated mice experienced noticeable gains in cognitive function."
额外的分析集中于事后人类brain tissue from Alzheimer's patients. These analyses showed that, similar to animals, levels of a specific carbonic anhydrase enzyme found in mitochondria are abnormally elevated in the brains of Alzheimer's disease and CAA patients. The evidence is the first to identifycarbonic anhydraseas a key factor in humans affected by these conditions.
The group plans next to design and investigate CAIs that are more specific to mitochondria, with the help of Marc Ilies, Ph.D., Professor in the Department of Pharmaceutical Sciences at the Temple School of Pharmacy.
"The therapies we used for the current study target carbonic anhydrases in the whole cell," Dr. Fossati explained. "If we can target the enzyme specifically within mitochondria, the efficacy of therapy could improve greatly, and side effects could be reduced."
Nonetheless,clinical trialsto test the effectiveness of acetazolamide and methazolamide against CAA in humans could be underway soon, as both agents are already approved by the FDA.
More information:Elisa Canepa et al, FDA‐approved carbonic anhydrase inhibitors reduce amyloid β pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness,Alzheimer's & Dementia(2023).DOI: 10.1002/alz.13063