This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Inflammation and cancer: Identifying the role of copper paves the way for new therapeutic applications
Inflammation is a complex biological process that can eradicate pathogens and promotes repair of damaged tissues. However, deregulation of the immune system can lead to uncontrolled inflammation and produce lesions instead. Inflammation is also involved in cancer. The molecular mechanisms underlying inflammation are not fully understood, and so developing new drugs represents a significant challenge.
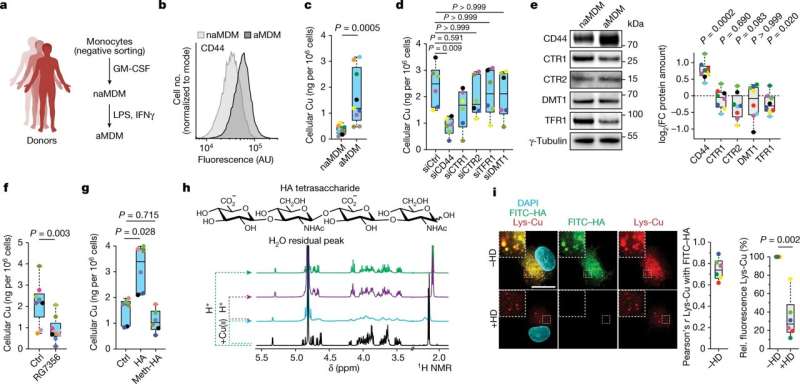
As far back as 2020, Dr. Raphaël Rodriguez, CNRS research director and head of the Chemical Biology team at Institut Curie (Equipe Labellisé Ligue Contre le Cancer) at the Cellular and Chemical Biology laboratory (Institut Curie/CNRS/Inserm), had shed new light on a membrane receptor called CD44, which marks immune responses, inflammation and cancer progression.
Dr. Rodriquez and his team showed that CD44 helped import iron into cell, triggering a series of reactions leading to activation of genes involved in the metastatic process. "This is a cell plasticity phenomenon we continued to study, investigating other metals potentially internalized by CD44, notably copper," he explains.
Copper causing epigenetic alterations
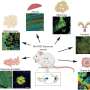
Along with his colleagues, Dr. Rodriguez has now reached a new milestone. The research team managed to identify a signaling pathway involving copper and leading to the expression of pro-inflammatory genes in macrophages, the cells present in all tissues and playing an important role in innate immunity.
Once internalized in macrophages, copper enters into the mitochondria (the organelle responsible for cell respiration andenergy production), where it catalyzes the oxidation of NADH into NAD+ (nicotinamide adenine dinucleotide, a molecule needed for the activity of certain enzymes). The increase of NAD+in cells enables the activity of certain enzymes involved in the production of metabolites essential for epigenetic regulation. These metabolites thus, contribute to the activation of genes involved in inflammation.
Inflammation and cancer: Shared molecular mechanisms
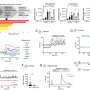
The scientists did not stop there, they also designed molecules able to bind to copper, inspired from the structure of metformin. By testing these new molecules on models of acute inflammation, they found that a synthetic dimer of metformin, LCC-12 (also termed Supformin), reduced activation of macrophages and attenuated inflammation. "Our work has enabled us to develop a drug prototype that inactivates copper chemistry in the cell's metabolic machinery, thus blocking expression of the genes involved ininflammation," explains Dr. Rodriguez.
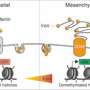
To finish, they applied this therapeutic strategy to cancer cell models engaged in an epithelial-mesenchymal transition. Here again, Supformin blocked the cellular mechanism and thus the cell transformation. "The genes activated in cancer cells are not the same as those expressed inimmune cells, but the chain reaction leading toepigenetic alterationsis identical," explains Rodriguez. These results thus reveal the role of copper incancer cellsand their ability to adopt a metastatic nature.
Dr. Raphaël Rodriguez concludes, "Our study reveals that the inflammatory and cancer processes depend on similarmolecular mechanismsand could therefore in the future benefit from similar innovative therapies, such as those tested with Supformin."
The paper is published in the journalNature.
More information:Stéphanie Solier et al, A druggable copper-signalling pathway that drives inflammation,Nature(2023).DOI: 10.1038/s41586-023-06017-4