This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Motor neuron disease treatments a step closer
Research at the University of Queensland could eventually help develop viable treatments—and ultimately a cure—for motor neuron disease (MND).
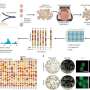
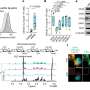
Dr. Adam Walker and co-authors Dr. Rebecca San Gil, Dr. Wei Luan and Ph.D. student Sean Keating from the Queensland Brain Institute have identifiedbiochemical changesin a protein that is affected by MND. Their research has been published inMolecular PsychiatryandCellular and Molecular Life Sciences.
"TDP-43 is a protein found in every cell of the body but is particularly important for the health ofmotor neurons, thebrain cellsthat control voluntary muscle movement," Dr. Walker said.
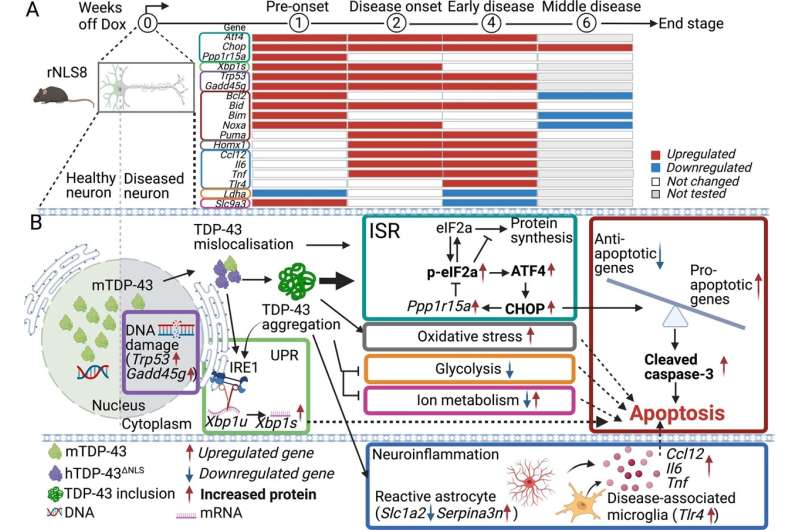
"We ran two research projects, looking at how TDP-43 proteins become dysfunctional in motor neurons.
"We found diseased versions of TDP-43 can damage healthy versions of the protein, which may create a cycle of protein dysfunction and degeneration over time.
"We also discovered that biochemical pathways which control neuron death are triggered early, even before MND symptoms begin.
"To change the course of the disease we need pharmaceutical drugs that can prevent neuron death and this TDP-43 protein dysfunction."
The research used genetic engineering technology called CRISPR, which is a gene editing tool.
"It allowed us to see TDP-43 inlive cellsfor the first time," Dr. Walker said.
Co-author Sean Keating said the research also found neural pathways change as MND progresses, indicating the potential need for different treatments at different phases of the disease.
"We are now treating genetically modified mice with MND with differentpharmaceutical drugsthat specifically target the underlying causes of the disease, and correct the disease mechanism," Mr. Keating said.
"Our aim is to stop the TDP-43 degenerative cycle and halt the progression of the disease.
“这项研究可以提高我们对MND的理解,and we hope it will play an important role in the fight against the disease."
More information:Wei Luan et al, Early activation of cellular stress and death pathways caused by cytoplasmic TDP-43 in the rNLS8 mouse model of ALS and FTD,Molecular Psychiatry(2023).DOI: 10.1038/s41380-023-02036-9
Sean S. Keating et al, Aggregation-prone TDP-43 sequesters and drives pathological transitions of free nuclear TDP-43,Cellular and Molecular Life Sciences(2023).DOI: 10.1007/s00018-023-04739-2