This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study shows NIH investment in new drug approvals is comparable to investment by pharmaceutical industry
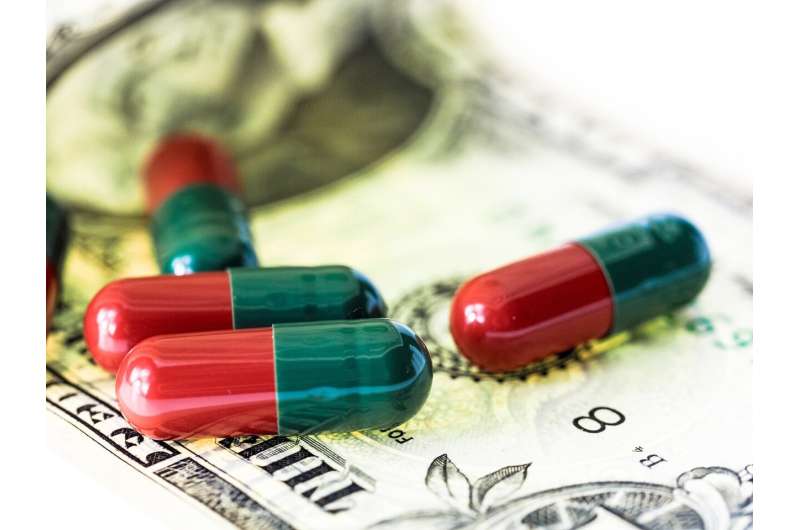
The National Institutes of Health (NIH) spent $187 billion for basic or applied research related to 354 of the 356 drugs approved by the FDA from 2010 to 2019, according to a new study from Bentley University's Center for Integration of Science and Industry. The study, published inJAMA Health Forum, shows that the amount invested per approved drug by the NIH is comparable to that of reported investment by the biopharmaceutical industry.
The article, titled "Comparison of research spending on newdrugapprovals by the U.S. National Institutes of Health versus industry, 2010-2019," is the first to compare the total value of NIH and industry investments taking into account actual spending on research related to approved products and failed product candidates as well as the time-value of these investments.
This paper estimated actual NIH spending of $1.4 billion for each approved first-in-class drug. The Tufts Center for the Study of Drug Development (CSDD) hasestimatedactual industry spending to be $1.5 billion per approved drug. Considering also a 3% annual discount, per druginvestmentby the NIH was $1.7 billion. NIH spending providedcost savingsto industry of $2.9 billion per approved drug (calculated with a 10.5% annual cost of capital), which is comparable to the Tufts CSDD estimate of $2.8 billion industry investment in each approved drug. This work shows the government served as an early investor in pharmaceutical innovations that are subsequently launched and commercialized by industry.
"Our analysis shows that at least half of the total investment in research and development required to bring a product to market comes from the U.S. government," said Fred Ledley, Director of the Center for Integration of Science and Industry, and the senior author on this study. "If taxpayers are investing as much as shareholders in bringing drugs to market, then the public could expect social or economic returns commensurate with those of pharmaceutical companies or their shareholders."
Industry has been criticized for high drug prices that make needed drugs unavailable to some patients. While industry claims that high drug prices are justified by the cost of bringing these drugs to market, the present work suggests that the public interest in these products should be balanced with corporate interest.
The Bentley study identified NIH funding for more than 400,000 research publications related to the drugs approved by the FDA from 2010 to 2019. Total NIHspendingwas $187 billion, with 83% of this total involving basic research on drug targets and 17% involving applied research on the drugs themselves. Statistical comparison of NIH investments in 60 drugs with industry costsreportedfrom the London School of Economics and Political Science show NIH investment was not less than industry investment.
这种分析还研究了经济efficiencies created by public sector funding for basic research that may provide a foundation for multiple product approvals. Considering that NIH-funded research on a validated drug target will be associated with an average of 2.85 drugs, the NIH invested an average of $711 million per drug approved 2010–2019.
More information:Comparison of research spending on new drug approvals by the U.S. National Institutes of Health versus industry, 2010-2019,JAMA Health Forum(2023).DOI: 10.1001/jamahealthforum.2023.0511,jamanetwork.com/journals/jama- … ealthforum.2023.0511