This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Study reveals a novel biomarker, potentially improved therapy for MS and related neurodegenerative disorders
Degeneration of myelin, an insulating sheath required for rapid communication between nerve cells, and neuroinflammation are notable hallmarks of Multiple Sclerosis (MS) and related neurodegenerative disorders such as Alzheimer's disease and Huntington's disease, which affect roughly 2.8 million people in the world. However, little is known about the precise molecular steps by which demyelination leads to the loss of neurons and glia, the two major types of brain cells.
A paper published recently inCell Metabolismby a research team led by Drs. Hugo J. Bellen, Hyunglok Chung, and Hyun Kyoung Lee at the Jan and Dan Duncan Neurological Research Institute (Duncan NRI) at Texas Children's Hospital and Baylor College of Medicine, reveals an answer to this longstanding question.
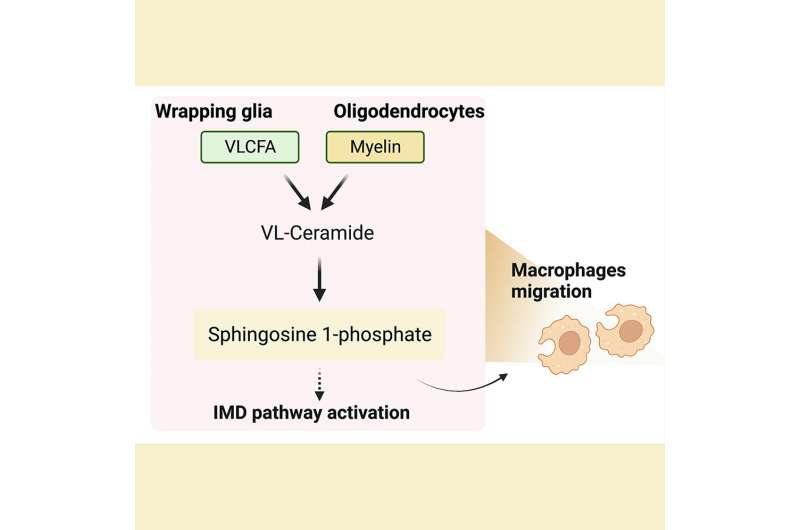
研究人员发现,髓磷脂分解results in an accumulation of very long-chain fatty acids (VLCFA) and their intermediates which triggers an autoimmune response that damages the brain cells. Furthermore, they showed that reducing the levels of VLCFA and S1P using known drugs, bezafibrate and fingolimod, had a synergistic beneficial effect on the MS pathologies in ananimal model, revealing an even more effective treatment for MS patients.
Elevated levels of S1P are toxic to fly glia and neurons
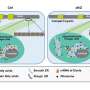
A previous study from the Bellen lab showed that the loss of the fly version of the dACOX1 gene reduced the lifespan, caused neuronal and motor dysfunction, and eventually resulted in the demise ofneuronsand glia. The ACOX1 gene encodes an enzyme required for the breakdown of VLCFA. In this study, the researchers set out to understand the exact molecular steps by which the absence of dACOX1 results in the loss of neurons and glia.
VLFCA are a rare group of fatty acids that comprise only a tiny fraction of the total fatty acids in the body. Myelin sheaths that surround the nerve membranes are a rich source of VLCFAs and have roughly 10-fold higher levels of VLCFA-ceramides than other cellular membranes. VLCFA are produced from long-chain fatty acids by ELOVL fatty acid elongase enzyme and converted back by the ACOX1 enzyme.
Dr. Chung, a former postdoctoral fellow in the Bellen lab who is also the first and co-corresponding author of this study, found that thetoxic effectsobserved due to the loss of dACOX1 could be suppressed by knocking down the gene that encodes ELOVL with bezafibrate, a lipid-lowering drug. These observations further supported their earlier observations that excess VLCFA is harmful tonerve cells.
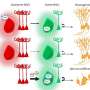
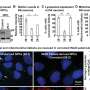
Dr. Chung and colleagues next assessed how increased levels of VLCFA in the glia affected the metabolism of other lipids. They performed a mass spectrometric analysis of 26 lipids obtained from adult fly heads that lacked the fly version of the ACOX1 gene. They found two lipid intermediates—very long ceramides(VL-Ceramides) and sphingosine-1-phosphate (S1P)—were significantly higher in the glia of these flies.
Further studies revealed that excess glial S1P was transported to the neurons and this increase in S1P levels was detrimental to the survival of both glia and neurons and was sufficient to cause these cells to malfunction and degenerate. Notably, they found that supplementing dACOX1 mutant flies with fingolimod, an MS drug known to bind and downregulate S1P receptor levels, led to dramatic improvements in the overall viability, neuronal function, and importantly, delayed neurodegeneration in these flies.
Together, their data provide compelling evidence that the accumulation of S1P, a key product of VLCFA catabolism, is the root cause for the demise of glia and neurons in dACOX1 mutants.
S1P triggers strong immune responses that destroy brain cells in flies
The strong suppression of neurodegenerative symptoms in dACOX1 mutant flies by fingolimod, a drug that is used to treat MS, an autoimmune disorder, promoted Dr. Chung to explore if elevated VLCFA had any impact on immune responses.
Intriguingly, he observed that flies lacking ACOX1 had several large black, melanotic masses throughout their body including the head, eye, wing margins, and abdomen. Typically, melanization is an immediate immune reaction deployed in arthropods like flies when they are attacked by pathogens and parasites. However, the presence of melanotic masses in these flies suggested that the absence of dACOX1 induces an autoimmune response whereby the immune cells misinterpret the presence of an innocuous molecule as a sign of a cellular invasion and mount an unwarranted attack that destroys their own cells.
They next asked if the loss of dACOX1 also activated other immune pathways.
Flies have two major immune pathways—the toll and the immune deficiency (Imd) pathways—that control inducible immune responses to invading bacteria and fungi by systemic production of cytokines, and antimicrobial peptides (AMPs) upon activation of the nuclear factor-kB (NF-kB).
Notably, the authors found that elevated S1P in flygliaactivates NF-kB which in turn significantly increased the transcript levels of several AMP genes involved in the IMD pathway. Moreover, circulating immune cells are recruited to the central nervous system.
"This is the first time this immune pathway has been shown to lead to neuroinflammation in adult fruit flies," said Dr. Bellen, a Baylor professor.
Bezafibrate and fingolimod ameliorate the progression of MS symptoms in mice
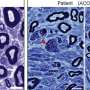
Excited by these findings, the team then explored the role of elevated VLCFA and S1P in MS progression in vertebrates by collaborating with Dr. Hyun Kyoung Lee, associate professor at Baylor and investigator at the Duncan NRI. The most commonly studied vertebrate model to study MS is the experimental autoimmune encephalomyelitis (EAE) wherein the mice are injected with myelin to induce MS-like pathology and immune responses.
First, a postdoctoral fellow in the Lee lab and co-first author, Dr. Qi Ye, found that pre-symptomatic treatment of these mice with bezafibrate, a lipid-lowering drug that inhibits the synthesis of VLCFA, slowed the progression of EAE pathology by reducingdemyelination, neuronal damage, and infiltration of immune cells into the brain. These results showed that this drug can slow the progression of this debilitating disorder.
She next tested the potential therapeutic effect of lowering VLCFA and S1P on MS. "When we administered bezafibrate along with fingolimod at the onset of symptoms, we saw a synergistic improvement in EAE-induced paralysis and motor performance, demyelination and neuronal loss. The combined effects of these drugs were significantly better than the effect of either drug alone in every parameter we tested—suggesting that a combined therapy will be more effective and offer better outcomes for MS patients," Dr. Chung added.
"We are very excited by the potential clinical implications of this study in not just how we treat MS patients but also for other neurodegenerative conditions that are associated with demyelination, disruptions in lipid metabolism, and neuroinflammation," Dr. Bellen said.
Dr. Chung plans to further explore the transport mechanism of S1P in his new role as an assistant professor in the department of Neurology at Houston Methodist.
Others involved in the study were Qi Ye, Ye-Jin Park, Zhongyuan Zuo, Jung-Wan Mok, Oguz Kanca, Sudhir Gopal Tattikota, Shenzhao Lu, Nobert Perrimon, and Hyun Kyoung Lee.
更多的信息:Hyung-lok涌et al, Very-long-chain脂肪酸induce glial-derived sphingosine-1-phosphate synthesis, secretion, and neuroinflammation,Cell Metabolism(2023).DOI: 10.1016/j.cmet.2023.03.022