This article has been reviewed according to Science X'seditorial processandpolicies.Editors强调了以下属性而在suring the content's credibility:
fact-checked
trusted source
written by researcher(s)
proofread
Sanofi vaccine: What to know about this protein-based COVID booster being offered in the UK

In the UK, aspring booster campaignhas recently begun, offering an additional vaccine to people at highest risk from COVID. Between April and June 2023 people aged over 75, residents in care homes and children and adults aged five and up with weakened immune systems will be invited to get their next shot.
For some this will be their sixth dose to date. But as immunity from COVID vaccinesbegins to wanein the months afterwards, this is a sensible precaution to help protect the most vulnerable against serious illness, hospitalization and death should they contract the virus.
During this vaccination campaign the UK will be using the updated bivalent vaccines from Pfizer and Moderna (which target omicron alongside the original COVID strain) as well as another type ofvaccinemade by Sanofi.
The Sanofi vaccine was approved for usein the UKandthe EUat the end of 2022. It's the seventh COVID vaccine to receive approval in the UK.
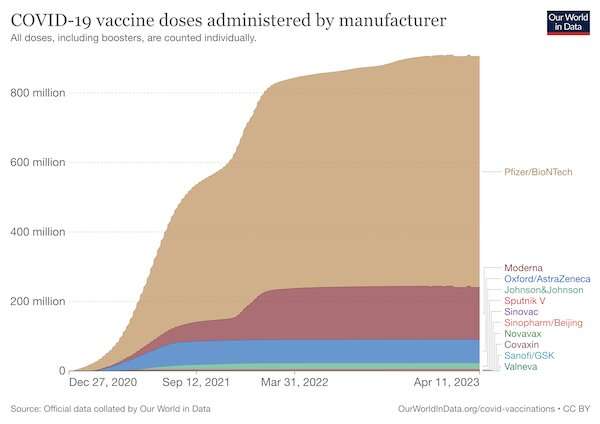
Over the past couple of years we've heard plenty about Pfizer and Moderna's mRNA-based vaccines. They've dominated the COVID vaccination campaign, particularly in high-income countries, withhundreds of millions of dosesadministered. But the Sanofi booster is based on a different vaccine technology, which hasn't been used before in a COVID booster campaign in the UK. So what is it, and how effective is this shot?
A protein-based vaccine
The Pfizer and Moderna vaccines contain mRNA, a small strand of genetic material that provides the instructions our cells need to make proteins. The mRNA instructs our cells to safely make copies of the spike protein which covers the surface of SARS-CoV-2 (the virus that causes COVID) and which helps it attach to and infect our cells. This trains our immune systems to recognize and kill SARS-CoV-2 if we encounter it.
The Sanofi vaccine, known asVidPrevtyn Beta, is protein-based and instead contains copies of the SARS-CoV-2 spike protein from the beta variant. This variant was first detected inSouth Africaand was classified as a variant of concern in December 2020.
So rather than instructing our cells to make copies of the spike protein as the mRNA vaccines do, the Sanofi vaccine contains copies of the spike protein already. Although the method is different, it delivers much the same effect—training our immune systems to recognize and kill SARS-CoV-2.
The Sanofi vaccine also contains an extra ingredient called an adjuvant, which stimulates our immune system to improve the vaccine's potency.
This type of vaccine design is not new. Several protein-based vaccines have been safely used for many years to provide immunity against the tetanus toxin as well as viral diseases such ashepatitis B,human papillomavirus,influenzaandshingles.
OtherCOVID vaccineshave also used this approach, including one made by Novavax thatwas approvedin the UK in February 2022. However, due to a number of reasons includingdelays in productioncompared to other manufacturers, the Novavax vaccine has not been used here.
Efficacy and safety
Early results from phase 3 trials indicate that the Sanofi vaccine, when used as a booster, is effective in generating strong virus-killingneutralizing antibody responsesagainst different COVID variants, including omicron. We call this cross protection, and it's important to help provide immunity against any future variants of concern.
How long the immunity from this vaccine may last is uncertain at this stage, but—in studies in animals—neutralizingantibody responses不同的变体是维护six months.
No serious adverse reactions or safety concerns have been reported in clinical trials.Side effects, if encountered, were generally mild to moderate and occurred within the first few days. These included headache, muscle and joint pains, fever, nausea and pain or swelling at the injection site.
Mixing and matching
Using different COVID vaccines at the same time will ensure sufficient stocks are available to complete the current booster campaign as quickly as possible.
If you're worried about getting a different vaccine to the one you had last time, there's no need. Mixing and matching the different types of vaccines used for the initial shots and subsequent boosters is known asheterologous vaccination. This has already been happening in the UK—many people will have had a mixture of AstraZeneca, Pfizer or Moderna vaccinations over the past two years.
Heterologous vaccination can actuallystrengthen and broadenthe immune response generated by the vaccine, helping to provide cross protection against different variants. In future booster campaigns we'll probably continue to see a range of different types of COVID vaccines offered.
Comparing the options
The simplicity of the mRNA vaccine technology has enabled these shots to be rapidly updated to deal with emerging variants such as omicron. Protein-based vaccines tend to takelongerto manufacture.
But they can be much cheaper to produce and can be stored in a refrigerator, unlike the mRNA vaccines which need to be stored in afreezer. These are useful characteristics to help improve vaccine equity in low and middle-income countries where vaccination coverage remainspatchy.
Making direct comparisons between different vaccines and their effectiveness is always difficult, but the Sanofi booster appears toperform wellwhen compared against themRNA vaccines.
如果你是医学上脆弱和提供a booster vaccination it's important that you take up this opportunity. Whether you're given a protein-based vaccine or an mRNA-based vaccine, each will provide a good boost to your immunity that will help to protect you against serious illness with COVID.
This article is republished fromThe Conversationunder a Creative Commons license. Read theoriginal article.![]()