This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How the tumor microenvironment stimulates pancreatic cancer growth and progression
Pancreatic cancers are deadly and hard to treat, in part because they are so often detected at an advanced stage; overall five-year survival rates are about 11%. Two separate labs at Boston Children's Hospital took out-of-the-box approaches to this difficult cancer, and both uncovered some very promising leads.
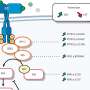
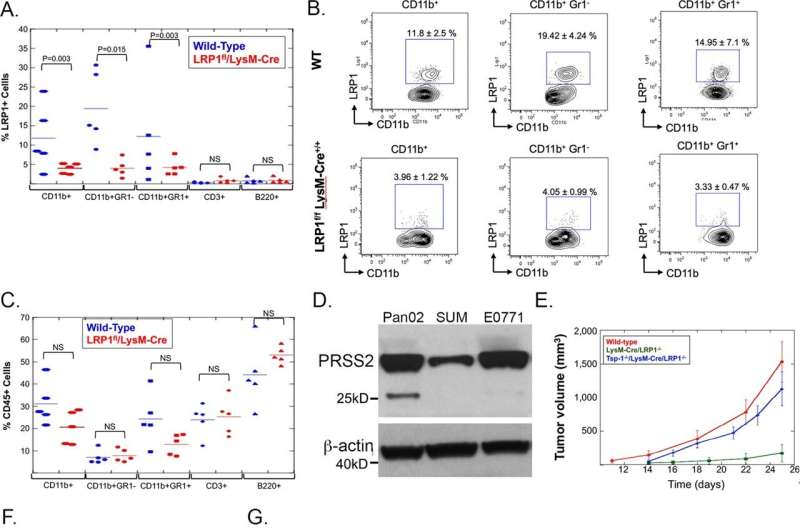
Randolph Watnick, Ph.D., an investigator in the Vascular Biology Program, started with the insight that immunotherapy, an emerging approach for a variety of cancers, has largely failed inpancreatic cancerbecause the tumor microenvironment—a protective "cocoon" surrounding the tumor—secretes multiple factors that block immune responses.
Watnick's team previously showed that a naturally occurring protein, thrombospondin-1 (Tsp-1), strongly inhibits growth of various tumors. They also showed that a compound called prosaposin, and compounds derived from it, can stimulate Tsp-1 to blocktumor growth. (Watnick has licensed these to Vigeo Therapeutics.) Butpancreatic tumorsare smart: They secrete a molecule called PRSS2 that suppresses Tsp-1.
Without Tsp-1, the tumor microenvironment throws up a slew of defenses that prevent theimmune systemfrom curbing the cancer. "These cells secrete so many agents that kill or inhibit activation of immune cells that it's hard to target all of them," Watnick says. "The tumor can grow without fear of the immune system."
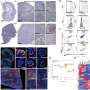
But when Watnick's team blocked PRSS2 in human cancer cell lines and in mice, they restored Tsp-1 expression, disabling many of the tumor's defenses in one stroke. This enabled the immune system to suppress tumor growth.
Even more exciting, PRSS2 circulates in the blood, making it an ideal candidate to target with antibodies. And because it doesn't normally mutate (it is merely over-expressed bytumor cells), the cancer is less likely to develop resistance to the treatment. This approach, described inNature Communications, might also work for ovarian andtriple-negative breast cancer, which have similar self-protection strategies, says Watnick.
"If we can make an antibody against PRSS2, it could potentially be a new lifeline for patients," he says. His lab is now working to develop an antibody and is considering strategies that would combine it with prosaposin and/or immunotherapies like PD1 inhibitors.
Starving pancreatic tumors
Nada Kalaany, Ph.D., an investigator in the Division of Endocrinology, took a completely different tack, asking the question, "What do pancreatic tumors need to grow?" She focused on pancreatic ductal adenocarcinoma, an aggressive cancer that relies on unusual metabolic pathways to obtain nutrients.
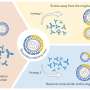
To support their growth, pancreatic tumors must make large amounts of compounds called polyamines. Kalaany's team showed that thetumor microenvironmentlacks a key ingredient, arginine, forcing the tumors to resort to anothermetabolic pathway. That pathway, predominantly used in infancy and in the fasting adult intestine, requires an enzyme known as ornithine aminotransferase or OAT.
“cancer overcomes a challenging, arginine-depleted microenvironment by using OAT to make polyamines," explains Kalaany, who is also an associate member of the Broad Institute. "If we target OAT, we can suppresstumorgrowth."
Indeed, when Kalaany's team inhibited OAT with a drug called 5-FMO, they curbed cancer growth both in a dish and in mouse models.
The findings,reported inNaturelast month, add another potential option for pancreatic cancer treatment. Targeting OAT avoids toxicity, since normal pancreatic cells don't need OAT to make polyamines. Since 5-FMO has been used experimentally for other purposes, Kalaany sees strong potential to translate the findings to the clinic.
"Cancers always figure out a way of getting around things," says Kalaany. "But if we target this pathway, they may not have a way to go around it."
更多的information:Lufei Sui et al, PRSS2 remodels the tumor microenvironment via repression of Tsp1 to stimulate tumor growth and progression,Nature Communications(2022).DOI: 10.1038/s41467-022-35649-9