This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Fewer vaccine doses still support pneumococcal immunity, shows clinical trial
A unique study published this month outlines new ways to protect more of the world's population from vaccine-preventable diseases.
The findings, detailed inThe Lancet Infectious Diseases, showed that administering fewer doses of a pneumococcal conjugatevaccine(PCV) could still protect against pneumococcal diseases such as meningitis and pneumonia.
Known as the Vietnam Pneumococcal Trial II, the randomized controlled trial was a collaboration between Murdoch Children's Research Institute, Pasteur Institute of Ho Chi Minh City and Menzies School of Health Research.
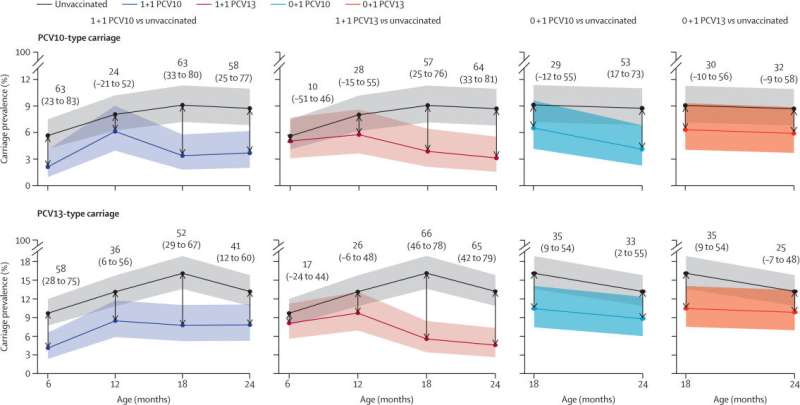
It showed that providing PCV doses to Vietnamese infants at 2 months and 12 months (known as a 1+1 vaccineschedule) reduced the presence of the pneumococcal bacterium found in the nose of healthy children (known as carriage) by up to two thirds.
The reduction in carriage reduces transmission and extends the protection from vaccination to others in the community. This is known as herd protection. The trial also saw an increase in individual immunity, helping to protect children in their first years of life.
Currently, 3 doses of PCV are recommended for infants. The Vietnam Pneumococcal Trial II adds to evidence suggesting a 1+1 vaccine schedule could be suitable in countries where pneumococcal vaccinations are already being implemented, or where they are yet to be used alongside catch-up campaigns. The United Kingdom has had a 1+1 PCV vaccine schedule since 2020, demonstrating the value of this approach.
The other key finding from the study was that one PCV dose provided at 12 months of age (known as a 0+1 vaccine schedule) could also provide some herd protection. This could help to reduce disease inhumanitarian crisesand remote settings where PCV access is challenging.
In bothvaccine scheduletypes, the 2 widely available PCVs were tested. Both vaccines were found to be effective at generating immunity and reducing carriage.
These findings, released ahead of World Immunization Week (24–30 April) highlight the action needed to promote the use of vaccines to protect people in all settings from disease.
Pneumococcal vaccines have been available since 2000, but it is estimated that 60% of children across the globe remain unvaccinated.
The Vietnam Pneumococcal Trial II has been made possible throughfinancial supportfrom the Bill & Melinda Gates Foundation.
According to Menzies School of Health Research Epidemiologist, Vietnam Pneumococcal Trial II Manager and lead author, Beth Temple, "Vaccination strategies that increase the accessibility and affordability of vaccines are urgently needed, and reduced vaccine schedules are one way to address this.
"If fewer doses can be given, this helps reduce the cost, logistics and resources, and quantity needed to vaccinate a wider group of people.
"The findings of the study provide substantial evidence to support the use of reduced-dose PCV schedules, and in turn can help protect more of our population against pneumococcal diseases."
Murdoch Children's Research Institute's Professor Kim Mulholland, Vietnam Pneumococcal Trial II Principal Investigator, stated, "Severe pneumonia, usually due to Streptococcus pneumoniae or pneumococcus, remains a major cause of child death in the poorer areas of the world. The children at highest risk are those living in poor, crowded housing, particularly those suffering from malnutrition.
"Sadly, 23 years after PCVs were first introduced, these children are still the most likely to miss out on the vaccine. A fair world would have seen to it that these children were vaccinated first. Our studies in this area are designed to help authorities to protect all children, not just those from wealthy families."
More information:Beth Temple et al, Efficacy against pneumococcal carriage and the immunogenicity of reduced-dose (0 + 1 and 1 + 1) PCV10 and PCV13 schedules in Ho Chi Minh City, Viet Nam: a parallel, single-blind, randomised controlled trial,The Lancet Infectious Diseases(2023).DOI: 10.1016/S1473-3099(23)00061-0