This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Worms as a model for understanding endocannabinoid system could inform better medications
If you give a worm some weed, he might just need a snack to go with it.
Worms exposed to a cannabinoid become even more interested in the kind of food that they'd already prefer, new UO research shows. The effect is similar to cravingpotato chipsand ice cream after a few puffs of marijuana—a phenomenon known scientifically as "hedonic feeding," but colloquially called "the munchies."
The study, led by neuroscientist Shawn Lockery in the College of Arts and Sciences, points to worms as a useful tool for understanding more about the many roles that cannabinoids naturally play in the body. And it could help researchers develop better drugs that target this system. He and his team published their findings April 20 inCurrent Biology.
Theendocannabinoid systemis a far-reaching signaling network that helps regulate key body systems like appetite, mood, and pain sensation. Molecules called endocannabinoids send chemical messages by interacting with cannabinoid receptors, special proteins that are sprinkled throughout the body and brain. Normally, these messages help keep different body systems in balance. But molecules in marijuana—like THC—also interact with cannabinoid receptors, making you feel high after partaking and causing other effects, too.
When Lockery and his team started this research, marijuana had just been legalized recreationally in Oregon, "so we thought, well heck, let's just try this!" said Lockery. "We thought it would be amusing if it worked."
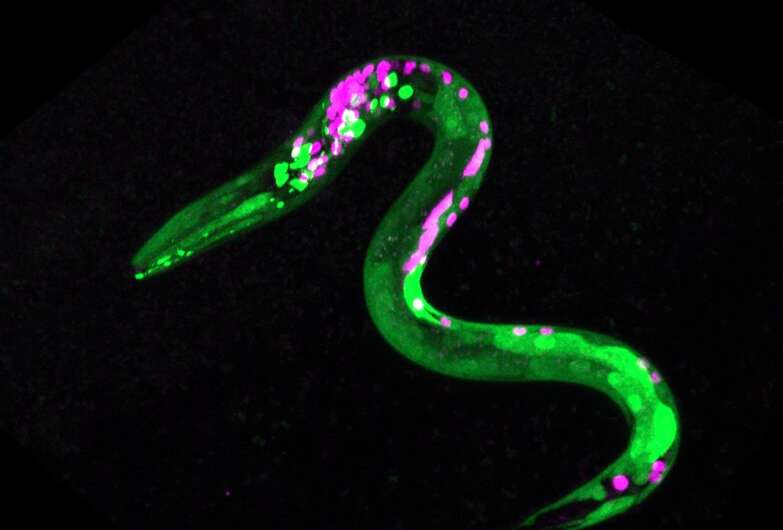
The idea wasn't totally out of left field. Research in the Lockery lab focuses on the neurobiology of decision-making, using a species of tiny bacteria-eating worms called C. elegans that eats bacteria as a simple system to test hypotheses. He often uses food choice experiments, tempting the animals with bacterial blends to see which they prefer under different conditions.
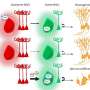
To see how marijuana-like substances might affect the worms' food preferences, Lockery's team soaked them in anandamide. Anandamide is an endocannabinoid, a molecule made by the body that activates the body'scannabinoid receptors.
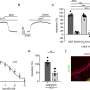
Then, they put the worms into a T-shaped maze. On one side of the maze was high quality food; on the other side, lower quality food. Previous research has shown that on high quality food sources, the worms grow quickly; on lower-quality ones, they grow more slowly. Worms also find high quality food more desirable, and preferentially seek it out.
In the T-maze experiment, under normal conditions, the worms indeed prefered the higher-quality food. But when soaked in anandamide, that preference became even stronger—they flocked to the high quality food and stayed there longer than they usually did.
"We suggest that this increase in existing preference is analogous to eating more of the foods you would crave anyway," Lockery said. "It's like choosing pizza versus oatmeal."
Higher quality food might call to mind a nutritious spread of fruits, veggies, and whole grains. But evolutionarily, "higher quality" food is the kind packed with calories to ensure survival. So in this case, "higher quality" worm food is more like human junk food—it packs in a lot of calories quickly.
"The endocannabinoid system helps make sure that an animal that's starving goes for high fat and sugar content food," Lockery said. It's one reason why, after consuming cannabis, you're more likely to reach for chocolate pudding, but not necessarily hungry for a salad.
In follow-up experiments, Lockery's team was able to identify some of the neurons affected by anandamide. Under the influence, these neurons became more sensitive to the smell of higher quality food, and less sensitive to the smell of lower quality food.
The results drive home just how old the endocannabinoid system is, evolutionarily speaking. Worms and humans last shared a common ancestor more than 600 million years ago, yet cannabinoids affect our food preferences in a similar way. "It's a really beautiful example of what the endocannabinoid system was probably for at the beginning," Lockery said.
The similarity in response between worms and humans also suggests thatwormscan be a useful model for studying the endocannabinoid system.
In particular, one current limitation with tapping into the medicinal properties of cannabinoids is their broad-ranging effects. Cannabinoid receptors are found throughout the body, so a drug targeting these receptors could help the problem at hand, but might also have lots of undesired side effects. For instance, smoking weed might relieve your pain, but could also make it hard to focus on work.
But the other nearby proteins that are also involved in the cascade ofchemical messagesvaries depending on the body system at play. So better drugs could aim at these other proteins, narrowing the effects of the drug.
Because scientists know so much about worm genetics, they're are a good study system for picking apart these kinds of pathways, Lockery suggests. "The ability to rapidly find signaling pathways in the worm could help identify-better drug targets, with fewer side effects."
更多的信息:Shawn R. Lockery et al, The conserved endocannabinoid anandamide modulates olfactory sensitivity to induce hedonic feeding in C. elegans,Current Biology(2023).DOI: 10.1016/j.cub.2023.03.013.www.cell.com/current-biology/f … 0960-9822(23)00301-9